Chemical Reactions of Haloarenes
Chemical Reactions of Haloarenes: Overview
This topic covers concepts, such as, Chemical Reactions of Haloarenes, Nucleophilic Substitution Reactions of Haloarenes, Fittig's Reaction & Wurtz-Fittig's Reaction etc.
Important Questions on Chemical Reactions of Haloarenes
In which of the following conversions oxidation is taking place
(i) propanone to propene
(ii) benzoic acid to benzaldehyde
(iii) bromobenzene to 1-phenylethanol
Benzyl chloride can be prepared from toluene by chlorination with –
The structure of the major organic product expected form the following reaction are
In the following reaction, the nucleophile will displace which of the halogen atoms most readily?
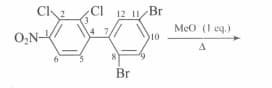
Examine the structural formula shown below and find out how many compounds undergo electrophilic nitration more rapidly than fluoro benzene.
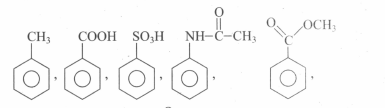
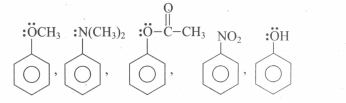
Assertion: In mono haloarenes, electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator.
Why does benzene undergo an electrophilic substitution reaction easily?
Explain electrophilic substitution reaction in haloarenes.
Electrophilic substitution reactions of Haloarenes are
Electrophilic substitution reactions of Haloarenes are meta directing.
Electrophilic substitution reactions of Haloarenes are
Write the mechanism of the electrophilic substitution reaction of Haloarenes.
Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards _____ substitution.(nucleophilic/electrophilic)
The halogen compound which will not react with phenol to give ethers is
The halogen compound which will not react with phenol to give ethers is
The rates of reaction of with:
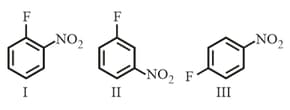
follow the order:-
The major product of the following reaction is _________
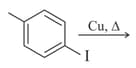
In the questions arrange the compounds in increasing order of rate of reaction towards nucleophilic substitution.
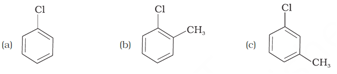
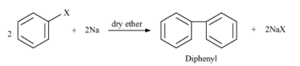
The above reaction is an example of _____ reaction. (Wurtz/Fittig)
Aryl halides are less reactive towards nucleophilic substitution reaction as compared to alkyl halides due to: